The Core Development
- Context: Gavi (The Vaccine Alliance) and UNICEF have finalized a landmark agreement to enhance the accessibility and affordability of the R21/Matrix-M malaria vaccine.
- Key Outcome: The deal slashes the vaccine price to $2.99 per dose, generating savings of $90 million.
- Impact: This financial efficiency will facilitate the procurement of 30 million additional doses, potentially immunizing nearly 7 million more children against malaria over the next five years.
- Financing Mechanism: The initiative is funded via an advance payment model backed by the International Finance Facility for Immunisation (IFFIm).
- Malaria Burden: African countries account for over 90% of the global malaria burden.
The Financial Backbone: What is IFFIm?
- Mechanism: An innovative financing tool that converts long-term pledges from donor governments into immediately available cash resources.
- Function: It issues “Vaccine Bonds” on capital markets, allowing Gavi to front-load funds for urgent immunization programs rather than waiting for annual aid disbursements.
Key Institutions in Focus
- Gavi, The Vaccine Alliance: A public-private partnership (PPP) focused on increasing access to immunization in poor countries. It facilitates procurement and market shaping.
- UNICEF: The world’s largest single buyer of vaccines, responsible for procurement and delivery logistics (supplying ~2 billion doses annually).
- Serum Institute of India (SII): The world’s largest vaccine manufacturer by volume; a critical partner in scaling the R21 vaccine for the Global South.
Malaria: Etiology and Preventive Strategy
Epidemiological Profile:
- Definition: An acute febrile illness caused by Plasmodium parasites.
- Vector: Transmitted exclusively through the bite of infected female Anopheles mosquitoes.
- Nature: Predominantly a tropical disease; it is life-threatening yet entirely preventable and curable.
- Transmission: Non-contagious (does not spread person-to-person).
Causative Agents & Symptoms:
- Parasitic Variants: While five species infect humans, Plasmodium falciparum and Plasmodium vivax pose the most significant public health threat.
- Incubation Period: Symptoms typically manifest 10–15 days post-infection.
- Clinical Presentation: Characterized by fever, headache, and chills.
- Asymptomatic Cases: Individuals in endemic regions may develop partial immunity, carrying the infection without visible symptoms.
Mitigation Strategies:
Vector Control: The primary line of defense.
Insecticide-treated nets (ITNs): Physical barriers treated with chemicals.
Indoor Residual Spraying (IRS): Application of insecticides to walls/surfaces where vectors rest.
Chemoprophylaxis: The administration of antimalarial medication to prevent infection in vulnerable populations.
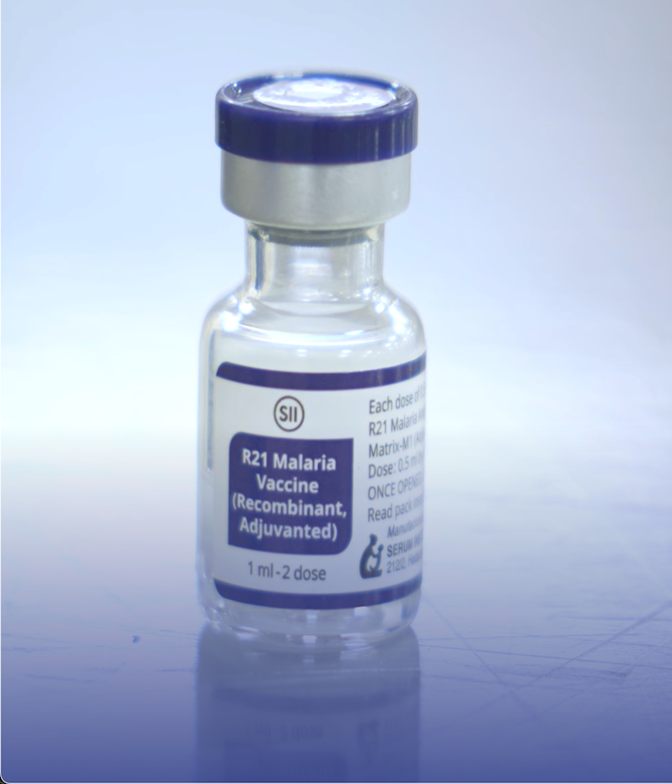
The R21/Matrix-M Vaccine
- Status: The second malaria vaccine to receive a WHO recommendation (following the RTS,S/AS01 in 2021).
- Collaborative Development: Designed by the University of Oxford and mass-manufactured by the Serum Institute of India (SII).
- technological Mechanism:
- Leverages Matrix-M, a proprietary saponin-based adjuvant developed by Novavax.
- Adjuvant Function: A substance added to vaccines to potentiate (enhance) the body’s immune response to the antigen.